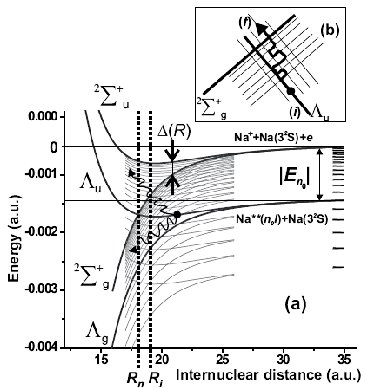
In order to develop understanding about elementary reactions involving atoms and molecules in Rydebrg states, we consider the simplest possible model process - a highly excited alkali atom colliding with an atom in the ground state: Na**(nl) + Na(3s). This kind of collisions can lead to the formation of the molecular ions via the process called ociative ionisation. To describe the process, we propose the stochastic ionization model [JPB 38, 1811 (2005)]. The model assumes that evolution of gases containing atoms in Rydberg states is strongly affected by stochastic processes accompanying interactions and collisions. Ionisation can be described in terms of stochastic diffusion of the valence electron through Rydberg states prior to the ionisation. Like the conventional theory [PRA 21, 819 (1980); JPB 14, 1639 (1981)], the model treats the ionization as excitation of Rydberg electron to the continuum by the electric dipole field generated by exchange interaction within the quasi-molecular ion, which is created during the collision. The stochastic model essentially extends this approach by taking into account redistribution of population over a range of Rydberg states prior to ionisation, which is caused by non-adiabatic processes in multiple overlapping level-crossings of quasi-molecular Rydberg states (see Fig. 5). As a result, the ionisation can occur at larger internuclear distances, than the earlier model suggests, and this leads to significantly altered ionisation rate constants. This is confirmed by recent experimental observations [JPB 38, 4349 (2005)]: at relatively low principal quantum numbers the stochastic ionisation model yields a substantially better agreement with the experimental data than the earlier models. The obtained results demonstrate the complexity of ionization processes following excitation of atomic Rydberg states, and indicate the path along which ionization of more complex systems involving molecules can be studied both experimentally and theoretically.